Author: Dr Payal Baheti
Plastic can either be ‘synthetic’ or ‘biobased’. Synthetic plastics are derived from crude oil, natural gas or coal. Whilst biobased plastics come from renewable products such as carbohydrates, starch, vegetable fats and oils, bacteria and other biological substances.
The vast majority of plastic in use today is synthetic because of the ease of manufacturing methods involved in the processing of crude oil. However, the growing demand for limited oil-reserves is driving a need for newer plastics from renewable resources such as waste biomass or animal-waste products from the industry.
In Europe, only a small proportion (about 4 – 6%) of our oil and gas reserves goes towards the production of plastics, with the rest used for transport, electricity, heating and other applications.
Most of the plastic in use today is derived by the following steps:
- Extraction of raw materials (largely crude oil and natural gas, but also coal) – these are a complex mixture of thousands of compounds that then need to be processed.
- Refining process transforms crude oil into different petroleum products – these are converted to yield useful chemicals including “monomers” (a molecule that is the basic building blocks of polymers). In the refining process, crude oil is heated in a furnace, which is then sent to the distillation unit, where heavy crude oil separates into lighter components called fractions. One of these, called naphtha, is the crucial compound to make a large amount of plastic. However, there are other means, such as using gas.
- Polymerisation is a process in the petroleum industry where light olefin gases (gasoline) such as ethylene, propylene, butylene (i.e., monomers) are converted into higher molecular weight hydrocarbons (polymers). This happens when monomers are chemically bonded into chains. There are two different mechanisms for polymerisation:
- Addition polymerisation
The addition polymerisation reaction is when one monomer connects to the next one (dimer) and dimer to the next one (trimer) and so on. This is achieved by introducing a catalyst, typically a peroxide. This process is known as chain growth polymers – as it adds one monomer unit at a time. Common examples of addition polymers are polyethylene, polystyrene and polyvinyl chloride.
- Condensation polymerisation
Condensation polymerisation includes joining two or more different monomers, by the removal of small molecules such as water. It also requires a catalyst for the reaction to occur between adjacent monomers. This is known as step growth, because you may for example add an existing chain to another chain. Common examples of condensation polymers are polyester and nylon.
- Compounding/Processing
In compounding, various blends of materials are melt blended (mixed by melting) to make formulations for plastics. Generally, an extruder of some type is used for this purpose which is followed by pelletising the mixture. Extrusion or a different moulding process then transforms these pellets into a finished or semi-finished product. Compounding often occurs on a twin-screw extruder where the pellets are then processed into plastic objects of unique design, various size, shape, colour with accurate properties according to the predetermined conditions set in the processing machine.
1. Polymer vs. plastic
All plastics are essentially polymers, but not all the polymers are plastics.
The term polymer and monomer are derived from Greek words: where ‘poly’ means ‘many’, ‘mer’ means ‘repeating unit’ and the word ‘mono’ means ‘one’. This literally means a polymer is made from many monomer-repeating units. Polymers are larger molecules formed by covalently joining many monomer-units together in the form of chains like pearls on a string of pearls.
The word plastic comes from ‘plasticus’ (Latin for ‘capable of moulding’) and ‘plastikos’ (Greek for ‘fit for moulding’). When we say plastics, we are referring to organic polymers (synthetic or natural) of high molecular weight which are mixed with other substances.
Plastics are high molecular weight organic polymers composed of various elements such as carbon, hydrogen, oxygen, nitrogen, sulphur and chlorine. They can also be produced from silicon atom (known as silicone) along with carbon; a common example is silicone breast implants or silicone hydrogel for optical lenses. Plastics are made up of polymeric resin often mixed with other substances called additives.
‘Plasticity ‘is the term used to describe the property, feature and attribute of a material that can deform irreversibly without breaking. Plasticity describes whether a polymer would survive the temperature and pressure during the moulding process.
Chemistry allows us to vary different parameters to tune the properties of polymers. We can use different elements, change the type of monomers, and rearrange them in different patterns to change the shape of polymer, its molecular weight or other chemical/physical properties. This allows plastics to be designed to have right properties for a specific application.
2. What are hydrocarbons?
Most plastic in use today comes from hydrocarbons derived from crude oil, natural gas and coal – fossil fuels.
What is a hydrocarbon?
Hydrocarbons are organic compounds (can be aliphatic or aromatic) made up of carbon and hydrogen. Aliphatic hydrocarbons have no cyclic benzene rings while the aromatics have benzene rings.
Carbon (C, atomic number = 6) has a valency of four, meaning it has four electrons in the outermost shell. It is able to pair up with four other electrons from any element of the periodic table to make up chemical bonds (for hydrocarbon, it will pair up with hydrogen). Hydrogen on the other hand (H, with atomic number = 1) has only one electron in the valence shell so four of these H-atom are ready to be paired up with C-atom by forming a single bond to give a C-H4 molecule. CH4 molecule is called methane, which is the simplest hydrocarbon and the first member of the Alkane family. Similarly, if two C-atoms would bond together they can link with up to six H-atoms with three being on each C-atom to give a chemical formula of CH3-CH3 (or C2H6) known as ethane and the series goes on as follows.
Alkane family: Methane (CH4), ethane (CH3-CH3 or C2H6), propane (CH3-CH2-CH3), butane (CH3-CH2-CH2-CH3), pentane (CH3-CH2-CH2– CH2-CH3), hexane, heptane, octane, nonane, dodecane, undecane and so on.
Note that this type of bond with carbon and hydrogen is a saturated bond (sigma bond denoted as σ-bond). There can also be unsaturated bond where a pi bond (π-bond) is present along with sigma bond giving carbon-carbon double bonds (alkenes) or have two π-bonds with a sigma giving carbon-carbon triple bond (alkynes), which very much depends on the type of hybridisation between the elements.
Alkene family: Ethylene (CH2=CH2 or C2H4), propylene (CH2=CH-CH2), 1-butylene (CH2=CH-CH2-CH3), 2-butylene (CH3-CH=CH-CH3) and so on. (Note that the 1-butylene and 2-butylene are isomers of butylene).
Alkyne hydrocarbons: Ethyne (CH ≡ CH or C2H2), propyne (CH≡C-CH3), 1-butyne (CH≡C-CH2-CH3), 2-butyne (CH3-CH≡CH-CH3) and so on.
What are fossil fuels and where do they come from?
Fossil fuels are mainly crude oil, natural gas and coal that are made up of carbon, hydrogen, nitrogen, sulphur, oxygen elements and other minerals (Figure 1). The generally accepted theory is that these hydrocarbons are formed from the remains of living-organisms called planktons (tiny plants and animals) that existed during the Jurassic era. The planktons have been buried deeper beneath the heavy layers of sediments in the Earth’s mantle, due to compression from an enormous amount of heat and pressure. Dead organisms decomposed without oxygen, which transformed them into tiny pockets of oil and gas. Crude oil and gas then penetrate in the rocks that ultimately accumulate in reservoirs. The oil and natural gas wells are found at the bottom of our oceans and beneath. Coal mainly originated from dead plants.
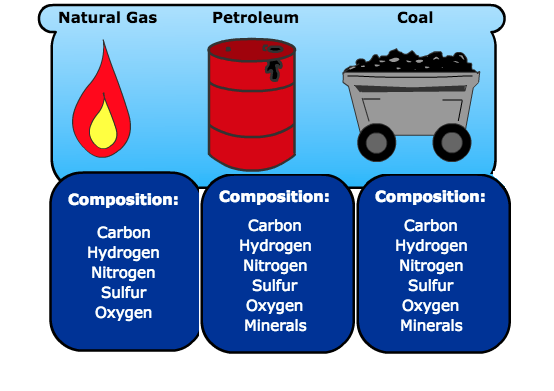
Figure 1. Elemental composition of fossil fuels.
Scientists have also questioned this theory. A recent study in Nature Geoscience from Carnegie Institution in collaboration with Russian and Swedish colleagues revealed that the organic matter may not be the source of heavy hydrocarbon and that they could be existing already deep down in the Earth. Experts discovered that ethane and other heavy hydrocarbons could be made if the pressure-temperature conditions can be mimicked with those present deep inside the Earths core. This is to say that hydrocarbons can be made in the upper mantle that is the layer of Earth between the crust and the core. They demonstrate it by subjecting methane to laser heat-treatment in the upper layer of the Earth that then transformed into hydrogen molecule, ethane, propane, petroleum ether and graphite. The scientists then exposed ethane to the same conditions which reversibility produced methane. Above findings indicate that these hydrocarbons might be created naturally without the remains of plants and animals.
- How is synthetic plastic created from crude oil?
Synthetic plastic comes from petrochemicals. When the source of oil beneath the surface of the Earth is identified, holes are drilled through the rocks in the ground to extract oil.
Extraction of oil – Oil is pumped from underground to the surface where tankers are used to transport the oil to the shore. Oil drilling can also take place under the ocean using support from platforms. Different size pumps can produce between 5 – 40 litres of oil per stroke (Figure 2).
Refining of oil – Oil is pumped through a pipeline that can be thousands of miles long and transported to an oil refiner (Figure 2). Spillage of oil from the pipeline during transfer can have both immediate and long-term environmental consequences but safety measures are in place to prevent and minimise this risk.
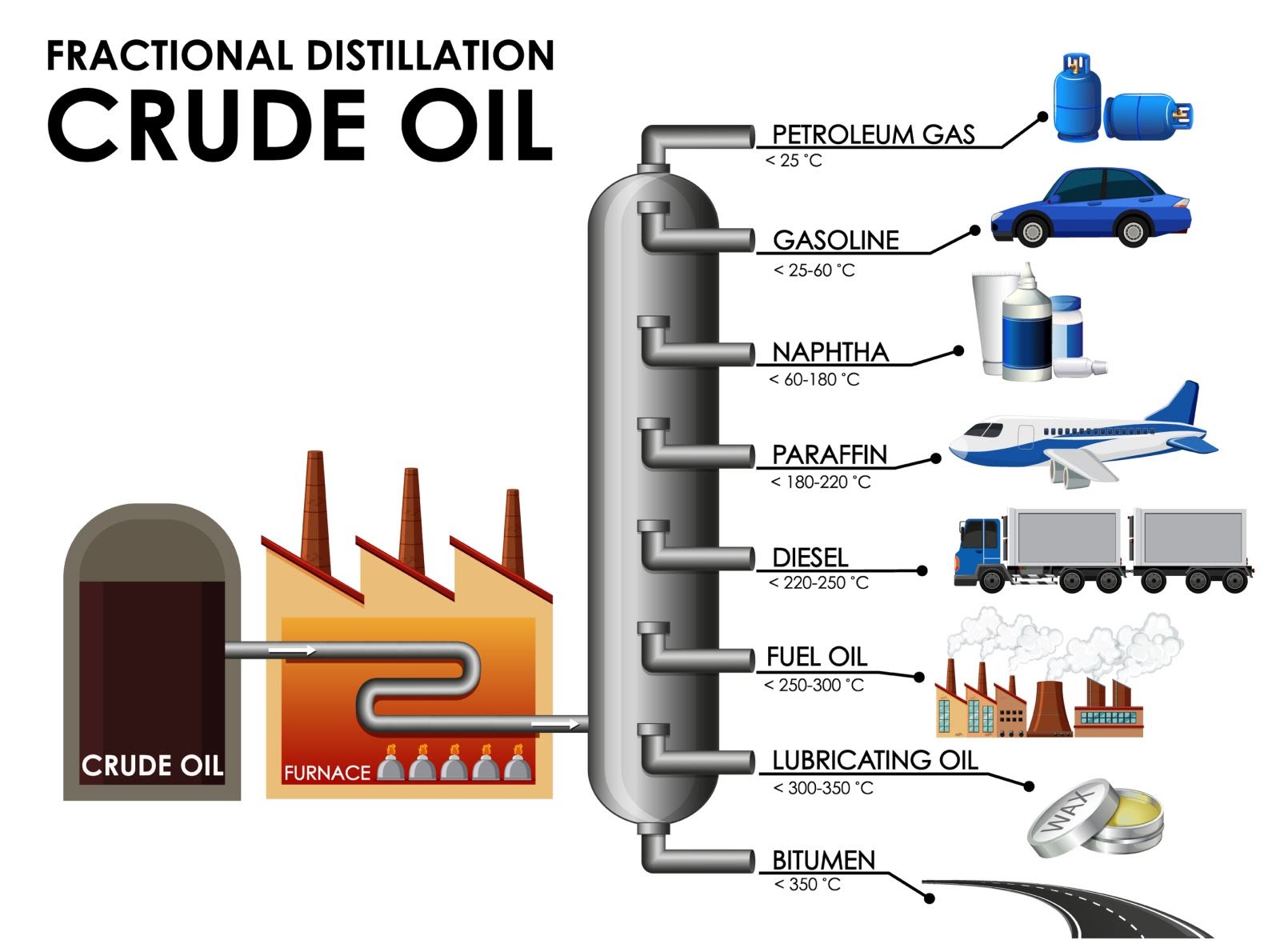
Figure 2: Fractional distillation of crude oil
Distillation of crude oil and production of petrochemicals – Crude oil is a mixture of hundreds of hydrocarbons that also contains some solids and some gaseous hydrocarbons dissolved in it from the alkane family (mainly it is CH4 and C2H6, but it can be C3H8 or C4H10). Crude oil is first heated into a furnace then the resultant mixture is fed as a vapour to the fractional distillation tower. The fractional distillation column separates the mixture into different compartments called fractions. There exists a temperature gradient in the distillation tower where the top is cooler than the base. The mixture of liquid and vapour fractions gets separated in the tower depending on their weight and boiling point (boiling point is the temperature at which the liquid phase changes into gaseous). When the vapours evaporate and meet a liquid fraction whose temperature is below the boiling point of vapor, it partly condenses. These vapours of evaporating crude oil condense at different temperature in the tower. Vapours (gases) of the lightest fractions (gasoline and petroleum gas), flow to the top of the tower, intermediate weight liquid fractions (kerosene and diesel oil distillates), lingers in the middle, heavier liquids (called gas oils) separate lower down, while the heaviest fractions (solids) with the highest boiling points remain at the base of the tower. Each fraction in the column contains hydrocarbons with a similar number of carbon atoms, smaller molecules are towards the top and longer molecules nearer the bottom of the column. In this way, petroleum is decomposed into petroleum gas, gasoline, paraffin (kerosene), naphtha, light oil, heavy oil, etc.
After the distillation step, the obtained long chain hydrocarbons are converted into hydrocarbons that can then be turned into many important chemicals which we use for the preparation of a wide range of products applicable from plastic to pharmaceuticals.
Cracking of hydrocarbon is the main process that breaks down the mixture of complex hydrocarbons into simpler low relative molecular mass alkenes/alkanes (plus by-products) by the means of high temperature and pressure.
Cracking can be performed into two ways: Steam cracking and catalytic cracking.
Steam cracKing uses high temperature and pressure to break the hydrocarbons long chains without a catalyst, whilst catalytic cracking adds a catalyst which allows the process to occur at lower temperatures and pressures.
The raw material used by the petrochemical industry is mainly naphtha and natural gas from oil refining operation in the petrochemical feedstock. Steam cracking uses the feedstocks from hydrocarbons mixture from various fractions such as reactant gases (ethane, propane or butane) from natural gas, or liquids (naphtha or gas oil) (Figure 3).

Figure 3: Various chemicals obtained from fossil fuel after oil refining.
(Naphtha is a mixture of C5 to C10 hydrocarbons obtained from the distillation of crude oil).
For example, decane hydrocarbon is cracked down into products such as propylene and heptane where the former is then used to make poly(propylene) (Figure 4).
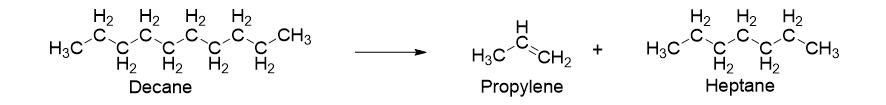
Figure 4. Representation of Cracking of decane to convert into propylene and heptane.
Raw materials molecules are converted into monomers such as ethylene, propylene, and butene and others. All these monomers comprise double bonds so that the carbon atoms can subsequently react to form polymers.
Polymerisation – hydrocarbon monomers are then linked together by chemical polymerisation mechanism to produce polymers. Polymerisation process generates thick, viscous substances as resins, which are employed to make a plastic product. If we look at a case of ethylene monomer here; ethylene is a gaseous hydrocarbon. When it is subjected to heat, pressure and a certain catalyst, it joins together into long, repeating carbon chains. These joined molecules (polymer) is a plastic resin known as polyethylene (PE).
Production of PE based plastic –poly(ethylene) is processed in a factory to make plastic pellets. The pellets are poured into a reactor, melted into a thick liquid to cast into a mould. The liquid cools down to harden into a solid plastic and produce a finished product. Processing of polymer also includes the addition of plasticizers, dyes and flame-retardant chemicals.
Types of polymerisations
Synthetic plastic is made by a reaction known as polymerisation, which can be performed in two different ways:
Addition polymerisation: Synthesis includes adding together monomers in a long chain. One monomer connects to the next and so on, when a catalyst is introduced, in a process known as chain growth polymers, adding one monomer unit at a time. Some addition polymerisation reactions are considered to create no side-products and the reaction can be performed in the vapour phase (i.e. gas phase) dispersed in a liquid. Examples: polyethylene, polypropylene, polyvinyl chloride and polystyrene.
Condensation polymerisation: In this case, two monomers combine to form a dimer (two units) by releasing a byproduct. Dimers can then join to form tetramers (four units) and so on. These byproducts are necessary to be removed for the success of the reaction. The most common byproduct is water, which is treated and disposed of easily. Byproducts can also be valuable raw materials that are recycled back into the feedstream.
Examples: Nylon (polyamide), polyester and polyurethane.
4. How is plastic created from naphtha?
Plastic is often created from naphtha. Ethylene and propylene, for example, are the main raw material for oil-based plastic coming from Naphtha.
What is Naphtha?
There are different types of naphtha. It is a term used to describe a group of volatile mixtures of liquid hydrocarbons, obtained by the distillation of crude oil. It is a mixture of C5 to C10 hydrocarbons.
Naphtha is decomposed thermally at high temperature (~800 °C) in a steam cracker in presence of water vapor where it splits into light hydrocarbons known as major intermediaries. These are olefins and aromatics. Among the olefins, there is C2 (ethylene), C3 (propylene), C4 (butane and butadiene). The aromatics consist of benzene, toluene and xylene. These small molecules are linked together by into long molecular chains called polymers. When a polymer comes out of the chemical factory they it is still not in the form of plastic – they are in the form of granules or powders (or liquids). Before they can become an everyday use plastic they need to undergo a series of transformations. They are kneaded, heated, melted, and cooled into objects of various shape, size colour with precise properties according to the processing tubes.
For instance, for polymerisation of ethylene into polyethylene (PE), initiators are added to start the chain reaction, only after the formation of PE, it is sent for processing by addition of some chemicals (antioxidants and stabilisers). After which an extruder convertsn PE into strings, thereafter grinders convert it into PE pellets. Factories then melt them into final products.
5. What is the main ingredient in plastic?
The main ingredient in most plastic material is a derivative from crude oil and natural gas.
There are many different types of plastics – clear, cloudy, solid colour, flexible, rigid, soft, etc.
Plastic products are often a polymer resin which is then then mixed with a blend of additives (See polymer vs. plastic). The additives are important as each of them are used to provide plastic with targeted optimum properties such as toughness, flexibility, elasticity, colour or to make them safer and hygienic to use for a particular application.
What type of plastic a product is made from can be sometimes be identified by looking at the number at the bottom of plastic containers. Some of the main types of plastic and the parent monomer is given below (Table 1). This table shows the types of plastic and the monomers that make up the plastic.
Table 1. Main polymer types, monomers and its chemical structures
| Resin identification code | Polymers | Monomers |
| ♳
PETE |
Polyethylene terephthalate (PET) | Ethylene glycol and Dimethyl terephthalate |
| ♴
HDPE |
High-density polyethylene
(HDPE) |
Ethylene (CH2=CH2)
*(lesser branching between polymer chains) |
| ♵
PVC |
Polyvinyl chloride
(PVC) |
Vinyl chloride (CH2=CH-Cl) |
| ♶
LDPE |
Low-density polyethylene
(LDPE) |
Ethylene (CH2=CH2)
*(excessive branching) |
| ♷
PP |
Polypropylene
(PP) |
Propylene (CH3-CH=CH2) |
| ♸
PS |
Polystyrene
(PS) |
Styrene |
| ♹
Others |
Other plastics including acrylic, polycarbonates, polylactic acid (PLA), fibres, nylon | Different monomers are used for a particular polymer.
For instance, PLA made from Lactic acid |
*The monomer used in LDPE and HDPE is ethylene but there is a difference in the degree of branching.
6. Which was the first human made plastic?
Meso American cultures (Olmec, Maya, Aztecs, 1500 BCE) used natural latex and rubber to make containers and clothes water-resistant.
Alexander Parkes (UK, 1856) patented the first man-made bioplastic, called Parkesine, made from cellulose nitrate. Parkesine was a hard, flexible and transparent plastic. John Wesley Hyatt (US, 1860s) made a fortune with Parkes’ invention. The Hyatt brothers improved plastic’s malleability of cellulose nitrate by adding camphor and renamed the plastic as Celluloid. The aim was to produce billiard balls, which until then were made from ivory. The invention is considered as the earliest example of man-made bioplastic by many.
The first truly synthetic plastic was Bakelite made from phenol and formaldehyde resin. Leo Baekeland (Belgium, 1906) invented Bakelite that was coined as a ‘National Historic Chemical Landmark as it completely revolutionized every industry present in modern life. It has the property of high resistance to electricity, heat, and chemicals. It has non-conducting properties, which is extremely essential when designing electronic devices such as radio and telephone casings.
7. What was used before plastic?
Before the birth of plastic, we were using wood, metal, glass and ceramic, and animal derived materials such as horn, bone and leather.
For storage purpose, mouldable clays (pottery) mixed with glass were used which meant the containers were often heavy and fragile.
Natural materials from the bark of the rubber tree – gum (latex resin) came into existence, the mix was sticky and mouldable but not useful for storage.
In the 18th century, Charles Goodyear accidentally discovered rubber – he added
In the 18th century, Charles Goodyear accidentally discovered rubber – he added sulphur into hot crude rubber that reacted and made rubber resilient which upon cooling became elastic i.e., it had the property to snap back into its original shape.
8. Can you make plastic without oil?
Yes, it is possible to create plastic from sources other than oil.
Although crude oil is the principal source of carbon for moden plastic, an array of variants are manufactured from renewable materials. Plastic made without oil is marketed as biobased plastic or bioplastics. These are made from renewable biomass such as:
- Lignin, cellulose and hemicellulose,
- Terpenes,
- Vegetable fats and oils,
- Carbohydrates (sugars from sugar cane etc)
- Recycled food waste
- Bacteria
However, it should be noted that bioplastics are not automatically a more sustainable alternative in every case. Bioplastics differ as per the ways in which they break down, and bioplastics also, as any material, require resources in their production.
Bioplastics such as PLA, for example, represent a biodegradable material that will degrade in certain environmental conditions, but may not bio-degrade in all sorts of climates. Therefore a waste stream of PLA based plastic is required. In the case of PLA it is a sensitive polyester that begins to degrade during the recycling procedure and can end up contaminating the existing plastic recycling stream.
But bioplastics can have many uses when designed with a proper waste stream in mind.
Bioplastics are potential materials for the fabrication of single-use plastic such as that required to make biodegradable bottles and packaging films. For instance, in 2019, a researcher from the University of Sussex created a transparent plastic film from fish-skin waste and algae; called MarinaTex. Biopolymers have also been investigated for medical applications, such as controlled drug release, drug packaging, and absorbable surgical sutures.
Maurice Lemoigne (France, 1926) discovered the first bioplastics made form bacteria, polyhydroxybutyrate (PHB), from bacterium Bacillus megaterium. As bacteria consume sugars, they will produce the polymers. The importance of Lemoigne’s invention was overlooked until the oil crisis hit in the mid-1970s spurred interest in discovering substitutes of petroleum-based products.
Henry Ford (US, 1940) used bioplastics made from soybeans for some car parts. Ford discontinued the use of soy plastics after World War II because of the surplus inexpensive oil supply.
The developments in metabolic and genetic engineering have expanded the research on bioplastic and applications for numerous types of bioplastics had become established particularly PHB and polyhydroxyalkanoate (PHA), although there are many other interesting developments occurring all the time.
